From HVAC and Facility design to Contamination Control Strategy Management
The update of the Eudralex Annex 1 (EUGMP) is coming soon and many companies of the health sector are already trying to prepare for it. In this update, Contamination Control Strategy appears to be a key concept to improve sterility insurance. But is it really a new approach? Do pharmaceutical companies need to start from scratch to be compliant with it? What are the roles of facility and HVAC on this subject?
In this article you will discover first level advisement to accompany your customer or your company on their journey to Annex 1 compliance.
Control Contamination Strategy Meaning
Let’s start with baseline requirements for room classification. The specifications about number of particles per size won’t change and remain as:
|
Grade |
Size |
EU at rest (part/m3) |
EU in operations (part/m3) |
US in operations (part/m3) |
Iso Class |
|
A |
≥ 0,5µm ≥ 5µm |
3 520 20 |
3 520 20 |
3 520 |
5 |
|
B |
≥ 0,5µm ≥ 5µm |
3 520 29 |
352 000 2 900 |
352 000
|
7 |
|
C |
≥ 0,5µm ≥ 5µm |
352 000 2 900 |
3 520 000 29 000 |
3 520 000 |
8 |
|
D |
≥ 0,5µm ≥ 5µm |
3 520 000 29 000 |
- |
- |
- |
First good news for pharma industries, as long as their environmental monitoring system is under control, they can make a reference to it into their CCS. In any case, it is a good opportunity to challenge the risks and the way rooms are monitored.
Nevertheless, contamination is not only a matter of particles even if they are the main vector for shipping virus, bacteria or fungi. Shortly linked to it, presence of human or any other living beings represents another high risk of contamination, so it is important to highlight in the CCS how to handle them and find solution to move them away as much as possible. Creating barrier as using isolator or RABS (Restricted Access Barrier System) is clearly highlighted in the annexe 1 (which is quite rare to get a solution described in the GMPs). On the other hand, using such equipment is not mandatory and getting a clear and factual rationales showing (with data) that contamination risks are under control is fully possible, probably more and more challenging but possible.
Second good news for pharma industries, they don’t necessarily need to change their main equipment, but they need to think objectively how new technology could help monitor and control the contamination risks. The recommendation would then be to perform and formalise technology watch and to include conclusion as part of the CCS. This is clearly a key aspect of a global continuous improvement strategy.
Moving the process in a fully closed system sounds quite impossible, so the way the building is designed and the way the HVAC system is functioning are also key on this battle and must be included in the CCS.
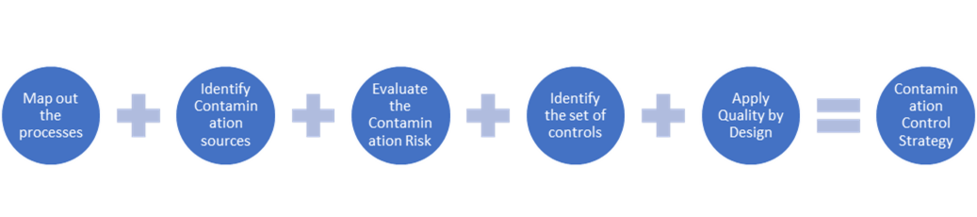
How to build a CCS
The PDA (Parenteral Drug Association) developed an approach to describe a CCS roadmap by following a step-by-step tool: Contamination Control Strategy: Implementation Road Map | PDA Journal of Pharmaceutical Science and Technology (paid article).
According to the PDA approach, inputs above and to move forward in a global approach, here is a flow to determine the CCS.