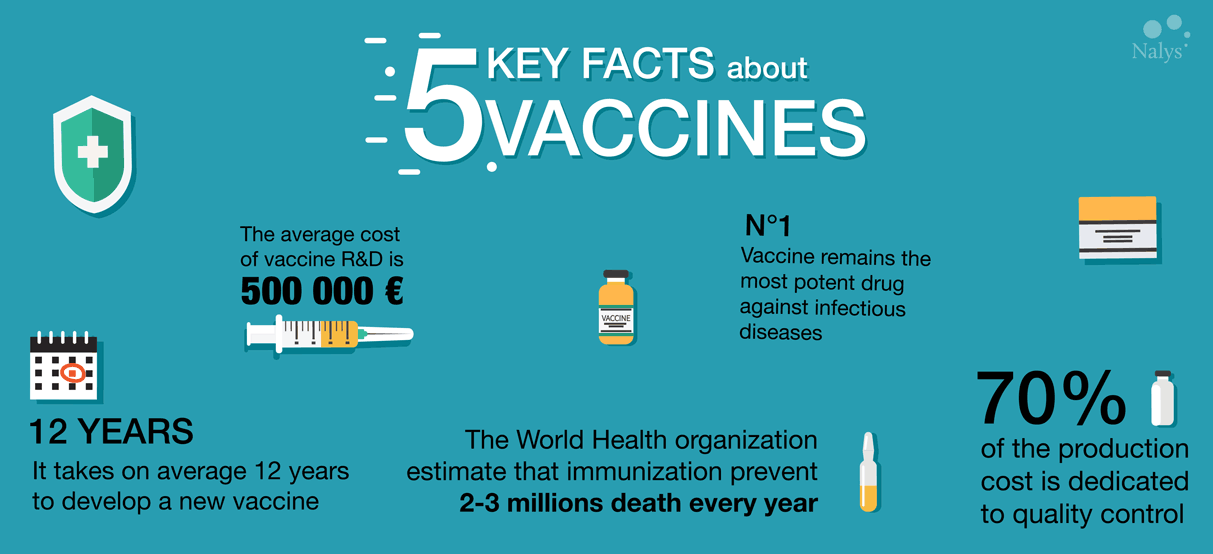
20 juin 2017
Technologie
What is a vaccine & what is its purpose?
The World Health Organiztion defines vaccine as a “a biological preparation that improves immunity to a particular disease”. The main purpose of a vaccine is to protect an individual against an infectious disease by preventing its development. But vaccination is also a way to protect an entire population against a disease. We call this effect the “herd immunity”. Its principle is simple: if the majority of people living in a community is immune to a disease, an unvaccinated person is less likely to get infected because there are less people to spread the disease.
A brief vaccine history:
In 1768, an English physician discovered that someone infected by cowpox (a harmful virus for cow but not for human), would become immune to smallpox (the “human & deadly version” of cowpox). Almost 30 years later, Edwar Jenner inoculate the cowpox virus to immune a little boy against smallpox, creating the very first vaccine. Since then, the smallpox has officially been eradicated and many vaccines have been creating following the same principle to cure other diseases. This process is still at the heart of many healthcare R&D projects and other innovations are to come.
Inactivated, attenuated or combined: the different types of vaccines:
If every vaccine follows a common purpose, the recipe to build an efficient treatment can be different. Today, there is 3 different ways of creating a vaccine
1. The attenuated vaccines: they are made up of living organisms (viruses, bacteria) that have been modified so that they lose their infectivity by retaining their ability to induce protection in the vaccinate person.
2. The inactivated vaccines: they don’t contain live infectious agent. They may contain either a fragment of the infectious agent or the whole of the infectious agent which is inactivated.
3. Combined vaccines: they are composed of several vaccine preparations collected in the same syringe.
HOW DO WE PRODUCE VACCINE?
STEP 1: CULTURE & PROLIFERATION OF VIRUS AND BACTERIAS
The first step of a vaccine creation is to “generate” enough bacteria and viruses, that will be used as material for the treatment.
For the bacteria: their multiplication needs to be put into cultivation in an environment conducive to its developments, this process is the FERMENTATION (exactly what is happening in the beer brewing ;))
For the viruses: their multiplication needs to put them in contact with cells in an environment conducive to their development. Virus infect cells and multiply. After the fermentation and the culture of viruses, the infectious agent is isolated from the culture medium.
STEP 2: PURIFICATION AND INACTIVATION
The purification allows to “clean” the infectious agent of the impurities of the culture media and the inactivation of the virus consists in ensuring that none of the infectious agent survive by means of physical or chemical methods.
STEP 3: FORMULATION (RECIPE)
The formulation consists of determining which “ingredients” to add to the inactivated infectious agent to retain its capacity to cause a reaction of the immune system without denaturing it.
STEP 4: DIVISION
There are 2 types of division: Syringe or vials (liquid or powder) Some vaccines (measles, rabies, yellow fever) are unstable in liquid form, they are lyophilized (= dehydrated). The powder will be reconstituted by diluent before administration to the patient.
STEP 5: QUALITY CONTROL & PACKAGING
When the syringes and vials are filled, they are inspected at the level of the contents (powder or liquid) and the container (vials or syringes). This step of control is the phase of mirage. It consists in visual inspecting the quality of the product with the naked eye and with the help of cameras (for example: absence of residues in vials or syringes, absence of cracks or micro-cracks in the containers …). When quality controls are validated, vaccines can be packaged and shipping.
STEP 6: SHIPPING
Vaccines are fragile biological substances that may lose their effectiveness when they are exposed to light or inadequate temperatures, their packaging must be carefully and respected. The storage temperatures of a vaccine are between 2 and 8°C. Temperature indicators can be placed on each folding box to ensure product compliance. The cold chain should not be broken throughout the life of the product.
Many thanks you to Marine, Sophie & Anne-Claire who help us to write this article :)
-
Librairie technique
21 févr. 2017
White paper: Environmental monitoring data
Life Sciences - Environmental monitoring (EM) program: a quality assurance tool.
-
Librairie technique
2 nov. 2016
White paper: Old production in the Life Sciences industry
Life Sciences - What is the biggest concern when working with old equipment?
-
Technologie
12 avr. 2017
3D printed organs: the future of medical devices
From the R&D to transplantation let’s have a look at the most amazing innovations in 3D printed organs.